The Pharmacy & Poisons Board is indeed a pharmaceutical and poison regulating authority created pursuant to Chapter 244 of its Law of Kenya, Pharmacy & Poisons Act.
The Board oversees opioid and poison practice, as well as drug manufacturing and trading. The Board is aimed at implementing effective regulatory mechanisms for ensuring the health of consumers, as envisaged by-laws on drugs in place in Kenya, to achieve the highest levels of security, effectiveness and efficiency for all drugs, chemicals and medical devices produced domestically, shipped, imported, marketed and then used.
PPB is a licensing and regulatory authority for the provision of relevant pharmaceutical facilities in Kenya. For one, you have to be licensed by the Pharmacy & Poisons Board to function in pharmacy. The internet resources of PPB are available to pharmaceuticals, veterinarians, pharmaceutical technologists as well as other medical professionals.
The licensing fee is extended annually for Pharmacists.
Vision of PPB
To be a world leader in public health promotion and security.
Mission of PPB
Protecting and encouraging public health by overseeing the medical profession and providing access to reliable, effective medical drugs and health technology in such a safe, accessible manner.
Core Values of PPB
Public health commitment, pace, integrity, professionalism, and teamwork
Mandate of PPB
The Drug Regulatory Authority formed in accordance with Chapter 244 of the Kenyan Pharmacy & Poison Laws seems to be the Pharmacy and Poisons Board.
The Committee governs pharmacy practices and drug and poison manufacturing and trade.
In order to provide consumer protection, as provided for under the rules of the drug in Kenya, the Board intends to enforce the required regulatory measures to meet the highest level of safety, effectiveness and consistency for all drugs, chemical substances and medical instruments domestically produced, imported, exported, distributed, marketed or even used.
The Drug Regulatory Authority formed in accordance with Chapter 244 of the Kenyan Pharmacy & Poison Laws seems to be the Pharmacy and Poisons Board. The Committee governs pharmacy practices and drug and poison manufacturing and trade.
In order to provide consumer protection, as provided for under the rules of the drug in Kenya, the Board intends to enforce the required regulatory measures to meet the highest level of safety, effectiveness and consistency with all drugs, chemical substances and medical instruments domestically produced, exported, imported, distributed, marketed or even used.
Core Functions of PPB
To safeguard and improve public health through the regulation of the pharmaceutical profession and ensure access to clean, effective and accessible pharmaceutical and healthcare technology.
Product Registration
Section 44(1) of the Pharmacy & Poisons Law, Cap 244 as well as misc. 2002 amendments to provide regulations under which drugs can be imported, made for sale or sold for Kenya enable the Minister of health in conjunction only with Pharmacy and Poison Board.
Pharmacy Practice
The Pharmacy & Poisons Board is committed to ensuring that pharmaceutical facilities are available in Kenya, which meet everyone’s needs in order to prevent, diagnose and cure diseases using healthy, reliable, high-quality and affordable pharmaceutical drugs.
Manufacturer Services
The Board retains the right to check the conformity of the producer to Good Manufacturing Practices only at the cost of the claimant.
Inspectorate
The wholesale and retail delivery of imported therapeutic products is part of the supply chain. Wholesalers are accountable for managing, storing and distributing those items effectively, effectively and safely.
Board Members
The members of the Board are
- Chairman – Medical Services Director
- Registrar – Chief Drug Administrator
- A veterinarian or surgeon appointed by him.
The board members shall be composed of
- Four designated pharmaceutical professionals of the Kenyan Pharmaceutical Association
- One from the Civil Service
- One from the Community Pharmacy
- One from the Pharmaceutical Industry
- One from the University Pharmacy School
- Pharmaceutical technician
- two Kenya Medical Association practitioners (KMA)
Services offered by PPB
Within the following programs, the Board offers:
- Assessment and registration of products. The Analysis certificates are also provided by national qualitative monitoring laboratory MEDS and by the Medicament Analyses and Research Unit of three approved laboratories;
- Assessment of Medicine and Medical Device Advertising Applications
- Ensure the best practice in manufacturing
- Pharmacist registration
- Pharmaceutical Technologists Registration
- Emitting annual licenses for practice
- Issue of Pharmaceutical Representatives Annual Permits
- Authorization of Pharmaceutical Training institutions Institutions
- Authorization of imports and exports of pharmaceuticals
- Pharmaceutical Registration/Outlets
- Post-market monitoring and monitoring of drugs
- Medicines and Pharmacy Documentation and Information Services
- Pharmacy Public Relations Programs
Functions of PPB Board
The Board’s duties as specified by statute are:
- Advising the Health Minister on both administrative and law enforcement issues.
- Make sure that almost all drug products produced in, imported or exported from the country meet the required specification, protection and effectiveness criteria and comply with specific codes of conduct and other regulations for staff, premises and practices working at production, promotion, procurement, storage, distributor and sale of all such products.
- Ensure that medicines comply continuously with defined requirements before supply to the end customer.
- To ensure the purchase, export, manufacture, stockpiling, distribution, sale or another handling of pharmaceutical products by properly approved persons.
- To issue licenses or authorizations, whether domestically made or imported only for national market or export, for medicinal drugs after proper evaluation.
- If such pharmaceutical drugs need to be revoked or removed from the market, their continuing use may adversely affect public health.
- To keep an inventory of medicinal products registered.
- Publication from period to period for public records of lists of approved medical drugs and products with marketing permissions.
- In order to make sure that only the applicants maintain up-to-date compliance records for medical drugs and accept alternatives.
- Both production facilities, importing officers, wholesalers, hospitals, clinics, marketers, pharmacies and retail stores should be inspected and licensed/authorized.
- To ensure the finished drugs released into the distribution chain are sampled and analyzed, and otherly tested to ensure conformity with labeled requirements
- Track the demand for the existence of illicit or falsified medicines.
- Ensuring product material, as approved by the Board, is used for promoting and selling medicinal drugs.
- To disseminate pharmaceutical goods knowledge to professions to support their ethical use.
- Monitoring and evaluation of the pharmaceutical law implementation.
- To counsel the Minister on issues relating to pharmaceutical goods regulation and registration.
- Amending laws and legislation in the light of the time requirement.
- Register pharmacists and keep the list of registered pharmacists until proper evaluation.
- To inscribe and retain the position of enrolled drug technicians after proper evaluation.
- For the intention of certification and retention throughout the PPB registry, inspect all the establishments teaching pharmaceutical programs.
Procedure for Pharmacists Registration and Pharmaceutical Technologists Enrollment
The procedure is performed by administering an assessment to individuals with degrees and diploma qualifications from the Pharmacy and Poisons Council recognized institutions. By each category, there are two categories of exams;
(i) Stage I & II for the pharmacist
(ii) Level I & II for Pharmaceutical Technologist
Stage I examinations
This applies to people graduating from universities outside Kenya. This applies. When you graduate, the pharmacy and poison board oversees you for just a one-year internship.
Stage II Examination
This is conducted after a one-year PPB and Stage I community internship completed by people from the University of Nairobi. Pharmacists who complete phase II examinations shall be entered throughout the Registry of Pharmacists.
Level I examination
This was given to individuals with a diploma at schools I outside Kenya and (ii) otherwise licensed by the Kenya Medical Training College Pharmaceuticals and Poisons board. When the examination is passed, a functional attachment under PPB is conducted for seven months.
Level II examination
This is for people who have completed the 7-month attachment for KMTC. The pharmaceutical technologist is inserted throughout the pharmaceutical technologist’s role after this is handed over.
License for Annual Practice
Registered pharmacists and registered pharmacy technologists have a yearly license to work lawfully. The submission is made by PPB with a specified form and published by the Department of Training and Assessment.
Guide for License Renewal and Registration
The following is a guideline to how students and practitioners need to be registered, logged into and renewed via a website of the Pharmacy & Poisons Board website.
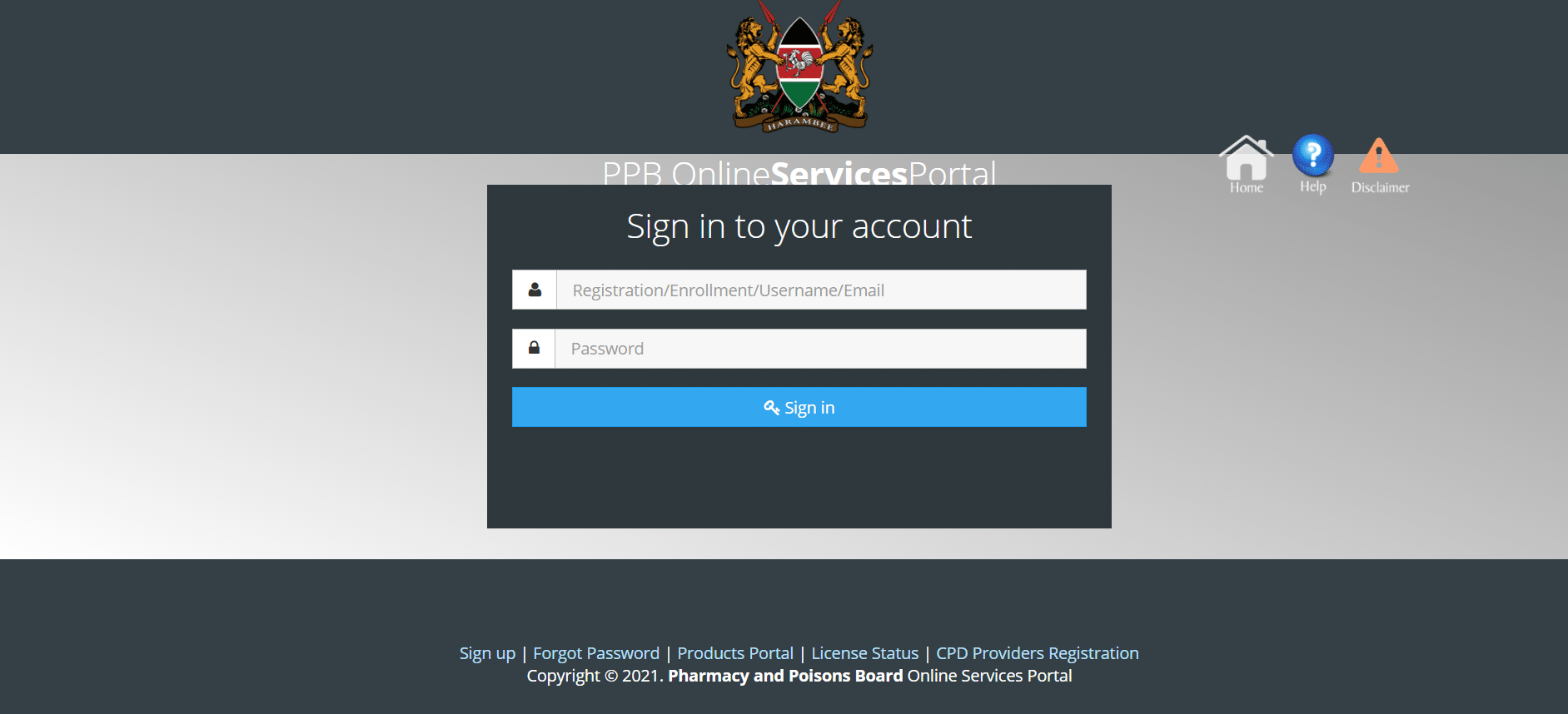
Registration Procedure of PPB portal
Following is the step-by-step guidance on accessing the site, creating an account, license renewal and several other online resources by the customer.
- Visit our website at the PPB portal to launch registration:
- Locate mostly on the website of the online services division
- Choose the services that you prefer.
- The GMP and Products tab could be specifically used by pharmacists and veterinarians. Other non-pharmaceutical staff and non-veterinarians must register in the Register again.
- By selecting mostly on the pop-up window of GMP and Products, you need to fill in your personal data, which is the Login ID and password. This applies only to pharmacists and vets.
- Many users who’ve been non-veterinarians and non-pharmacists and in this situation should select the data in the login window, which opens a tab to fill out their data.
- Your business name, KRA pin, postal address, identification code, contact information (email address and phone number), company name and their details will be required for the system.
- Your academic records, a duplicate of your ID card, as well as KRA diploma, KRA certificate business and company CRI 12 must be uploaded.
- The fields are automatically filled in for the method in the portion of the payments. It will also show the date of request, payment date, acceptance date as well as the name of the approved officer.
- To proceed, click the submit button. The framework would show the relevant areas that need to be updated in case of incorrect details.
- The user must complete the registration by generating new login credentials on the very same tab.
- Complete your login information with the email you got earlier. Enter and validate a password throughout the spaces given for your selection.
- To complete your registration, please click on the Acceptance terms and conditions button.
- You will get an email and will also be eligible for device entry with your login ID.
Guide for License Renewal
You must take the following steps to update your consumer license as just a pharmacist, pharmacy technologist or vet.
- Go to the www.pharmacyboardkenya.org website of PBP.
- Resources available online Click the License Portal for Practice
- You would be forwarded to a page where your identification number and password would be used to log in.
- You must build an account for new subscribers by pressing the login button.
- You are expected to select a setting on the registration window page: Pharmacist, Pharmtech, Pharm Rep and Veterinarian.
- For pharmacists, they shall fill out their registration number, the registration number of pharmacists and pharmacists, when they first got their card from the commission, shall really be the PBP number they were given.
- Please enter your national identification number and include the correct email address.
- Press the button Register
- A link to the tour email would be sent to you to complete the registration.
- When you click on the key, the new user password would be accessed in your profile tab.
- Mostly on the Profile Edit tab, click. Tap on the PBP portal to access the homepage.
- Click on the alternative on this page, Renovation.
- Fill in the window page with your info. Give your name, building name, mode of payment, as well as superintendent.
- The following records must be uploaded; KPA or PSK, prior license.
- Click Confirm and continue.
Process for Payment
- Press on the button Payments
- You will get an invoice amount mostly on the window of the payment.
- Select the option payment and enter 826826
- Please enter the invoice number as that of the account number issued
- Enter the number, confirm the information and click mostly on money
You would access your license renewal document after paying the full amount.
Final Words
So this was all in the guide to the PPB portal. So if you are a pharmacist or pharmacy technologist, then get yourself registered with the PPB board Kenya to get the license.